Taxon Attribute Profiles

On Cooper's Creek near Innamincka, Sth Aust.
|
Eucalyptus camaldulensis Dehnh.
River Red Gum
Introduction
Eucalyptus camaldulensis is a common and widespread tree along
watercourses over much of mainland Australia. It is frequently a dominant
component of riparian communities, and is an iconic and important species
of the Murray-Darling catchment, both ecologically and economically.
Taxonomy and Ecology
Classification
Family: Myrtaceae
Genus: Eucalyptus- c. 800 species, with all but three or four
endemic to Australia.
Subgenus: Symphyomyrtus
Notes: Eucalyptus camaldulensis exhibits considerable morphological
variation throughout its range, and consequently a number of infraspecific
taxa have been described. Var. camaldulensis is the most widespread,
and the only one occurring in the Murray-Darling Basin. For further discussion
on morphological variation, see Brooker et al. (2002). Chemical
and genetic variation has also been recorded in E. camaldulensis
(e.g. see Doran and Brophy, 1990; Stone and Bacon, 1994; Butcher et
al., 2001).
Life form
Eucalyptus camaldulensis is a perennial, single-stemmed, large-boled,
medium-sized to tall tree to 30 m high (Bren and Gibbs, 1986), although
some authors (e.g. Boland, 1984; Brooker et al., 2002) record trees
to 45 m. According to Jacobs (1955) river red gum could reach ages of
500 to 1000 years. See Brooker et al. (2002) for further descriptive
information.

Trees in floodwater at Hattah Lakes, Vict. |

Trees on dry creek bed east of Broken Hill, NSW |
click to enlarge map
|

Eucalyptus camaldulensis fringing the Murray River, Tooleybuc,
NSW. (Photo: J. Palmer) |
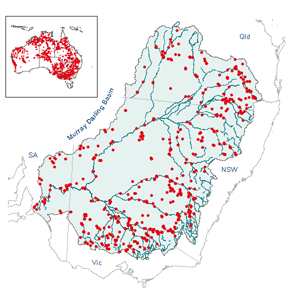
Distribution
Eucalyptus camaldulensis is found over most of the Australian
mainland, except southern Western Australia, south-western South Australia
and the eastern coastal areas of Queensland, New South Wales and Victoria
(Chippendale, 1988). It is widespread along rivers of all continental
Australia (Brooker and Slee, 1996). The accompanying map shows distribution
within Australia as well as in the Murray-Darling Basin.
Habitat
Eucalyptus camaldulensis commonly grows on riverine sites, whether
of permanent or seasonal water (Brooker et al., 2002). It is most
extensive on grey heavy clay soils along river banks and on floodplains
subject to frequent or periodic flooding, preferring deep moist subsoils
with clay content (Costermans, 1989). It also lines the channels of sandy
watercourses and creeks (Boland, 1984), commonly forming ribbon stands
but sometimes extending over extensive areas of regularly flooded flats.
It can also occur in the higher reaches of creeks in major valleys of
hilly country (Cunningham et al., 1981) and infrequently on the
margins of salt lakes (CAB International, 2000).
In the Murray region it is most commonly found on brown and red clays
(Dalton, 1990), and in the Chowilla area it is found along the main Murray
River channel and along the backwaters and billabongs (Roberts and Ludwig
1990, 1991).
"Status" in community
Eucalyptus camaldulensis is generally dominant in the community,
commonly forming pure open forests or woodlands (Costermans, 1989). On
lower levels of the floodplain, it is usually the only tree species present.
On higher areas, it may occur in association with black box (Eucalyptus
largiflorens) in the south or coolibah (E. microtheca) in the
north (Dalton, 1990).
Associated species
Eucalyptus camaldulensis is recorded as occurring with a variety
of other tree, shrub and herb species throughout its extensive range,
and these are not considered further in this profile.
At Chowilla, Roberts and Ludwig (1990, 1991) recorded E. camaldulensis
as a dominant species of two riparian communities: "River red gum
and reed community" (E. camaldulensis primarily with Phragmites
australis), and "River red gum and sedge-rush community"
(E. camaldulensis primarily with mixtures of Eleocharis, Juncus,
Cyperus and Cynodon dactylon)
At Chowilla, E. camaldulensis was recorded in three main communities
in a survey undertaken during 1988-1989 (see O’Malley and Sheldon,
1990).
- "Floodplain Black Box ± Red Gum ± Lignum ± River Cooba –
Forb Communities" on clay-based soils, on low undissected floodplain,
oxbow, channel edges and levee banks.
- "Red Gum Forest Communities" comprising dense red gum forest
with forb ± sedge ± grass understorey or floating freshwater aquatic
herbland, with fringing semi-aquatic forbs, sedges and grasses in billabongs.
Found on anaerobic clay on the low dissected floodplain.
- "Weedy Lagoon Communities", on grey cracking clays of an
intermittent lake, were fringed by red gum open forest with an herbaceous
understorey. This was an apparently disturbed site with high proportion
of exotic species.
Qualitative and quantitative data – abundance, cover, biomass
No specific data are available relating to cover, abundance or biomass.
As noted above E. camaldulensis is a dominant tree in the landscape.
Species – interactions with other biodiversity
River red gum forest wetlands provide habitat for fish and waterbirds
(breeding, feeding and refuge areas). This requires a certain length of
flooding duration and time of year. Hollows and spouts in river red gum
provide habitat for water and forest birds, including two rare species
of parrot (Superb Parrot (Polytelis swainsonii) and Regent parrot
(Polytelis anthopeplus)) in the Murray River region (Dalton, 1990).
Forty-nine phytophagous insects were collected from E. camaldulensis
canopies at Gulpa Island State Forest in 1991 and 1992 (Stone and Bacon,
1994). High levels of defoliation have been observed during outbreaks
of Uraba lugens (gumleaf skeletoniser) (Dalton, 1990) and Doratifera
spp. (cup moths).
Mistletoe infestations tend to be localised and occur in stands already
stressed by drought or insect attack. Tree death usually only occurs in
severe cases (Dalton, 1990).
Physiological traits and adaptations
Trees possess deep sinker roots, hypothesised to grow down towards zones
of higher water supply (Bren et al., 1991). These roots have extremely
high rates of hydraulic conductivity, making them very effective in conducting
water (Heinrich, 1990).
Seedlings can develop aerenchymatous roots to cope with immersion (see
Juvenile period and seedling survival below).
It has been suggested (Chesterfield et al., 1984; Chesterfield,
1986 cited in McEvoy, 1992) that the relatively low species richness underneath
E. camaldulensis stands in the Barmah forest may be a result of
allelopathic suppression from the overstorey. However, others suggest
it may be a result of flooding regimes and water stress (see McEvoy, 1992).
Leaf shedding reduces water demand by reducing leaf area. It also reduces
heat load under dry conditions when transpiration is reduced (Gibson et
al., 1994 in Roberts, 2001).
Reproduction and Establishment
Reproduction
Breeding system
The eucalypt breeding system is one of mixed mating with preferential
outcrossing. Although eucalypts are commonly self-compatible, self-pollination
generally results in a reduction in capsule production, seed yield and
seedling vigour (see House, 1997). Analyses of the breeding system of
E. camaldulensis indicate a predominantly outcrossing mating system
(CAB International, 2000).
Pollination
Pollination is mainly by insects but also by birds and small mammals
(CAB International, 2000).
Flowering
Eucalyptus flowers in most years from late spring to mid-summer
(July to February according to Brooker and Kleinig, 1999, December to
February according to Boland, 1984). Flowering intensity is variable and
unpredictable from year to year. About 45% of flowers fail to mature (Dexter,
1978).
Fruit/seed
Fruit development and maturation time can be as short as four months
(CAB International, 2000). Number of viable seeds per unit weight of a
seedlot : mean 698,000/kg (http://www.florabank.org.au/support/articles/sowingtheseeds.doc).
Eucalyptus species store little or none of their seed in the soil
(see McEvoy, 1992).

Eucalyptus camaldulensis fruit.
©EUCLID
|

Eucalyptus camaldulensis seed.
©EUCLID
|
Establishment
Eucalyptus camaldulensis is a free producer of seed. Dense stands
of young plants appear over extensive areas after floods, at times forming
impenetrable thickets. These saplings gradually thin out as they grow
(Cunningham et al., 1981). See section on Juvenile period below
for more information.
In Eucalyptus species, passive release of seed is aided by wind
(House, 1997). Free seed fall is least during winter and greatest in spring
and summer. High seed fall in spring may have adaptive significance as
floods usually recede during this period (Dexter, 1978). Eucalyptus
camaldulensis seeds sank within 36 hours of being dropped into still
water in laboratory tests and it was suggested that under field conditions
they would sink more rapidly (Dexter, 1978). However, McEvoy (1992) found
that seeds remained buoyant for at least 17 days after sowing. He suggested
that there might be a potential for floodwaters to act as a dispersal
agent.
Dispersability; establishment and growth

Regenerating fringe of E. camaldulensis at Purda Billabong,
near Wentworth, NSW.
(Photo: J. Roberts, 2002). |
Saplings gradually thin out as they grow, to form forests of straight-trunked
trees. In more arid regions, where ribbon stands occur along creeks, the
tree is more gnarled and develops a large spreading canopy. This form
also occurs throughout the region wherever the tree grows in isolation
on deep fertile soils with a good moisture supply. Eucalyptus camaldulensis
has probably one of the fastest growth rates for a tree and with a good
water supply can attain a height of 12-15 m in a few years (Cunningham
et al., 1981). Competition for moisture by ground vegetation and
/or overstorey trees can influence seedling survival depending on seasonal
conditions and flooding. The availability of moisture is greatly reduced
within the zone of influence of trees (which may extend to 40 m around
a mature tree). In the absence of competition seedling survival is 20-30
times greater (Dexter, 1978).
Juvenile period
Generation time may be as short as three years from planting to the production
of the first seed crops (CAB International, 2000). Precocious flowering
may occur as early as six months (Khan, 1965, cited in House, 1997). For
wild trees the time to first flowering is more likely to be five years
for a few scattered individuals and 7-10 years for general flowering.
The seed from early flowerings is usually very disappointing in terms
of germination capacity and seedling growth, probably reflecting high
levels of inbreeding (pers. comm., J. Doran, 2004).
Fruit development and maturation time can be as short as four months
(pers. comm., J. Doran, 2004).
Seedling establishment rather than germination is the critical stage
in stand regeneration.
Flood timing affects germination success. Flood recession in spring-early
summer is optimal for regeneration while winter floods with winter recession
are unfavourable. Spring-summer floods followed by summer recession provide
suitable germination conditions but subsequent heat and water stress can
cause massive seedling mortality. Germination can happen without flooding
if the winter is wet. If seedlings survive frost, but conditions continue
dry, moisture stress in the following summer is likely (Roberts and Marston,
2000).
Seedlings are vulnerable during the establishment phase to heat stress
and immersion. Seedlings cope with heat stress by developing roots giving
good penetration into the sub-soil and accessing soil moisture. Seedlings
also develop resilience early, allowing them to shed leaves in times of
moisture stress and recover from axillary buds when moisture is again
available (Dexter, 1978).
Seedlings develop adventitious roots and aerenchymatous tissue to deal
with anoxia resulting from immersion (Heinrich, 1990). Complete immersion,
unless brief, is likely to kill seedlings; lower leaves of small saplings
die if submerged for long periods (Roberts and Marston, 2000).
Seedlings increase tolerance to flooding with age. Two-month old seedlings
can survive waterlogging for one month (Marcar, 1993), while seedlings
50-60 cm tall can survive extended flooding of 4-6 months and complete
immersion for a few weeks by shedding leaves (Dexter, 1978).
The ability of the species to compete with weeds is poor when young (pers.
comm., J. Doran, 2004).
Hydrology and salinity
Hydrology
Eucalyptus camaldulensis obtains its water from three main sources:
ground water, rainfall and river flooding. It is river flooding which
enables the species to survive in semi-arid areas.
The unregulated flooding regime in western New South Wales consisted
of peak flows in late winter and spring with low flows in summer and autumn
(Dalton, 1990). Changes in the river flow patterns of the Murray, as a
result of large scale dam building, has led to reduced extent and depth
of winter flooding, reduced frequency of flooding, increased duration
of non-flood periods, increased occurrence and variability of summer floods,
increased river flow capacity (as a result of desnagging) and decreased
total annual flow. These changes have produced major deterioration in
much of the riparian forest, including reduced tree growth rate, accelerated
mortality and minimal regeneration (Bacon et al., 1993).
Stands of river red gum are intimately associated with the surface-flooding
regime of the watercourses and related ground water flow. The high water
use of river red gums contributes to maintaining watertables at depth
(Dalton, 1990).
Stabilised water levels are characteristic of large parts of the Chowilla
floodplain, (Roberts and Ludwig, 1991). At the time of the Chowilla floodplain
biological study (O'Malley and Sheldon, 1990) there was a high incidence
of dieback amongst river red gum and black box woodland associations.
It appears that woodland areas removed from the main channel or anabranch
creeks are more susceptible to dieback. Dieback is variously attributed
to altered hydrologic regime (reduced frequency and depth of floodplain
inundation) or increasingly saline soils (due to mobilisation of saline
groundwater as a consequence of the hydraulic pressure exerted by the
lock).
At Chowilla the two riparian communities described by Roberts and Ludwig
(1990) were found in two distinct places. The river red gum and sedge-rush
community occurred in riparian habitats where current was slow and the
bank was gently sloping and not subject to strong wave action. The river
red gum and reed community was associated with relatively fast currents
and steep banks exposed to strong wave action.
Regeneration of river red gum was recorded at several channel edge localities,
especially where the channel bank was not far elevated from the anabranch
creek level (O'Malley and Sheldon, 1990).
Before the introduction of regulation on the Murray River, groundwater
in the Chowilla region flowed under the floodplain into the river. After
the installation of locks, which also resulted in the previously ephemeral
creeks being continuously filled with water, the direction of groundwater
flow was reversed. The groundwater now discharges into the anabranch creeks
and into the soil (Jolly and Walker, 1995).
Most recharge of the groundwater system at Chowilla is dominated by flooding.
The heavy clay soils in the area also act to decrease the impact of rainfall.
Three types of flood recharge occur. ‘Bank recharge’ is when
a stream recharges the aquifer through the bank. ‘Diffuse recharge’
is the recharge of the groundwater through the soil surface after the
stream has broken its banks. In ‘localised recharge’ the floodwater
infiltrates through isolated areas of the floodplain at a higher rate
(e.g. in old depressions, dunes with a thin clay layer or old meanders).
See Jolly and Walker (1995) for a discussion on the different impacts
of the three types of recharge.

Dead Eucalyptus camaldulensis in severely salt effected landscape
on Reny Island near Renmark, Sth. Aust. (Photo: M.Fagg 1995) |
Salinity tolerance
Eucalyptus camaldulensis demonstrates moderate salt tolerance
(Benyon et al., 1999). Benyon et al. (1999) showed that
increasing salinity is associated with reduced tree growth in an experiment
on a saline discharge site near Wellington, NSW. Growth was better for
E. camaldulensis trees planted on non-saline soil than on moderately
saline soil. An increase in soil salinity was associated with a decrease
in the average leaf area per tree.
Thorburn et al. (1994) showed that river red gums in the Chowilla
floodplain were not obtaining all their water from the creek, even when
the trees were over highly saline groundwater. Eucalyptus camaldulensis
trees at Chowilla that only had access to surface water during a flood
were not utilising low-salinity floodwaters in preference to more saline
groundwater during a flood period (Thorburn and Walker, 1994). Results
indicated that the trees might be less affected by changes in creek flow
and/or salinity than was previously thought (also see Thorburn et al.,
1992; Mensforth et al., 1994).
Flooding regimes
The change in the river flow has led to a decline in river red gum health
and changes in the understorey composition. Permanent inundation leads
to river red gum death (Dalton, 1990). However, river red gums have survived
relatively long periods of continuous flooding (24 months at Barmah and
3-4 years behind the Hay Weir (Bren, 1987)). Field observations suggest
river red gums can survive 2-4 years of continuous flooding before showing
signs of stress (Roberts and Marston, 2000).
Forest flooding, particularly in late winter, is a key factor in controlling
the leaf skeletoniser moth by: providing conditions favourable to the
growth of a fungal pathogen of the insect (Aspergillus); removing
pupation sites within the ground litter; drowning the insect larvae. Reduction
of flooding frequency through regulation has advantaged these insects
(Dalton, 1990).
Change in water regimes
Reduced flooding has resulted in less water being available for regeneration
and seasonal growth. Permanent inundation results in tree death. Other
vegetation communities have adapted to infrequent flooding and are able
to expand, usually at the expense of river red gum communities (Dalton,
1990).
Eucalyptus camaldulensis was seen to be ‘invading’ a
natural grassland in the Barmah-Millewa Forest, presumably as a result
of changes in river regulation (Bren, 1992)
Response to disturbance (non-hydrological)
Grazing
Rabbits and kangaroos heavily graze seedlings during prolonged dry periods
when feed is scarce (Dexter, 1978). Until 1950s grazing of river red gum
forests was at a fairly high level, modifying the original understorey
(Dalton, 1990). Where narrow bands of trees occur along a watercourse,
too high grazing pressure will disadvantage maintenance of a self-replacing
stand. In these cases only a small amount of regeneration results, and
this is easily grazed out by stock. However, sapling growth is not, or
rarely, grazed by stock unless animals are starved of other forage (Cunningham
et al., 1981). Cattle grazing on weeds may help control weeds,
reducing competition for moisture (Dexter, 1978).
Fire
Eucalyptus camaldulensis is very fire sensitive and even low intensity
fires may cause cambial injury (Dexter, 1978). Fire kills regeneration
and even mature trees are susceptible if the fire is intense enough since
E. camaldulensis lacks a lignotuber. Fire will cause damage to
the butt, lowering the value of the timber and predisposing tree to fungal
and insect attack (Dalton, 1990).
Other
Feral pigs can disturb large areas through digging and wallowing, causing
erosion and destroying wetland areas (Dalton, 1990).
Conservation status
Eucalyptus camaldulensis is one of the most widespread tree species
across Australia, and is not considered at risk.

Scarred bark of an Aboriginal
'canoe tree'.
|
Uses (including ethnobotanical)
River red gum forests are historically and culturally important due to
the number of significant Aboriginal sites they contain. Common relics
include canoe and shield trees. Such trees show scars where the bark was
removed (Dalton, 1990).
The wood has been used for heavy construction, railway sleepers, flooring,
framing, fencing, plywood and veneer manufacture, wood turning, firewood
and charcoal production (Boland, 1984).
Eucalyptus camaldulensis is of major importance in Australia as
a source of honey, producing heavy yields of nectar in good seasons (Clemson,
1985). It also provides bees with an important source of good quality
pollen (CAB International, 2004).
Due to its natural adaptation to both temperate and tropical climates
with both winter and summer rains, river red gum is the most widely planted
species in arid and semi-arid regions around the world, primarily in timber
plantations (Eldridge et al., 1993 in CAB International , 2000).
Summary
Compared with most species, there is a considerable bank of knowledge
relating to Eucalyptus camaldulensis and its functioning in the
landscape, and in particular its performance in the Murray-Darling Basin.
From past changes in water regimes we know that E. camaldulensis is
affected by changing water levels and that mature stands have been lost
through permanent flooding. Flood timing affects germination success,
and seedling establishment, the critical stage in regeneration, is vulnerable
to heat stress and immersion. Eucalyptus camaldulensis is not physiologically
adapted to either drought or salinity, although these stresses can be
tolerated for short periods or at low levels.
Stands of river red gum are associated with the surface flooding regime
of watercourses and related ground water flow. The species is a profligate
and opportunistic water user, and this is a contributing factor to the
maintenance of water tables at depth. Even without large amounts of empirical
data it is clear that loss of large tracts of the species in the Murray
River corridor would have a major impact on the hydrology of the system,
as well as on vegetation communities and associated biodiversity.
References
Bacon, P.E., Stone, C., Binns, D.L., Leslie, D.J. and Edwards, D.W. (1993)
Relationships between water availability and Eucalyptus camaldulensis
growth in a riparian forest. Journal of Hydrology 150, 541-561.
Benyon, R.G., Marcar, N.E., Crawford, D.F. and Nicholson, A.T. (1999)
Growth and water use of Eucalyptus camaldulensis and E. occidentalis
on a saline discharge site near Wellington, NSW, Australia. Agricultural
Water Management 39, 229-244.
Boland, D.J., Brooker, M.I.H., Chippendale, G.M., Hall, N., Hyland, B.P.M.,
Johnston, R.D., Kleinig, D.A. and Turner, J.D. (1984) Forest Trees of
Australia. Nelson and CSIRO, Melbourne.
Bren, L. (1990) Red Gum Forests. In Mackay N. and Eastburn, D. (eds)
The Murray. Murray-Darling Basin Commission, Canberra, 230-242.
Bren, L.J. (1987) The duration of inundation in a flooding river red
gum forest. Australian Forest Research 17, 191-202.
Bren, L.J. (1992) Tree invasion of an intermittent wetland in relation
to changes in the flooding frequency of the River Murray, Australia. Australian
Journal of Ecology 17, 395-408.
Bren, L.J. and Gibbs, N.L. (1986) Relationships between flood frequency,
vegetation and topography in a river red gum forest. Australian Forest
Research 16, 357-370.
Brooker, M.I.H. and Kleinig, D.A. (1999) Field Guide to Eucalypts, South-eastern
Australia. Volume 1, Bloomings Books, Hawthorn.
Brooker, M.I.H. and Slee, A.V. (1996) Eucalyptus. In Walsh, N.G.
and Entwisle, T.J. (eds) Flora of Victoria, Volume 3, Dicotyledons: Winteraceae
to Myrtaceae, Inkata Press, Melbourne.
Brooker, M.I.H., Connors, J.R., Slee, A.V. and Duffy, S. (2002) EUCLID:
eucalypts of southern Australia (CD Rom), CSIRO Publishing, Collingwood.
Butcher, P.A., Otero, A., McDonald, M.W. and Moran G.F. (2001) Nuclear
RFLP variation in Eucalyptus camaldulensis Dehnh. from northern
Australia. Heredity 88: 402-412.
CAB International. (2000) Eucalyptus camaldulensis. Forestry Compendium
Global Module. CAB International, Wallingford, UK.
Chesterfield, E.A. (1986) Changes in the vegetation of the river red
gum forest at Barmah, Victoria. Australian Forestry 49, 4-15.
Chippendale, G.M. (1988) Flora of Australia, Volume 19, Myrtaceae, Eucalyptus,
Angophora. Australian Government Publishing Services, Canberra.
Costermans, L. F. (1989) Native trees and shrubs of south-eastern Australia,Weldon,
Sydney.
Cunningham, G.M., W.E.Mulham, P.E.Milthorpe and J.H.Leigh (1981) Plants
of Western New South Wales, Soil Conservation Service of New South Wales.
Dalton, K. (1990) Managing our river red gums. Soil Conservation Service
of New South Wales, Sydney.
Dexter, B.D. (1978) Silviculture of the River Red Gum forests of the
central Murray floodplain. Proceedings of the Royal Society of Victoria
90, 175-194.
Doran, J. and Brophy, J.J. (1990) Tropical gums – a source of 1,8-cineole-rich
Eucalyptus oil. New Forests 4, 157-178.
Heinrich, P. (1990) The eco-physiology of riparian River Red Gum (Eucalyptus
camaldulensis) Final Report, Australian Water Resources Advisory Council.
House, S.M. (1997) Reproductive Biology of Eucalypts in Williams, J.
and Woniarski, J. (eds). Eucalypt ecology: individuals to ecosystems.
Cambridge University Press.
Jacobs, M.R. (1955) Growth Habits of the Eucalypts. Forestry and Timber
Bureau, Canberra
Jessop, J.P. (1986) Myrtaceae. In Jessop, J.P. and Toelken, H.R., Flora
of South Australia: Part II, Leguminosae – Rubiaceae, South Australian
Government Printing Division, Adelaide.
Jolly, I.D. and Walker, G.R. (1995) A sketch of salt and water movement
in the Chowilla floodplain, CSIRO Division of Water Resources.
McEvoy, P.K. (1992) Ecophysiology of 3 Eucalyptus species on the
River Murray floodplain. Unpublished thesis, M.For Sci, Univeristy of
Melbourne.
Mensforth, L.S., Thorburn, P.J., Tyerman, S.D. and Walker, G.L. (1994)
Sources of water used by riparian Eucalyptus camaldulensis overlying
highly saline groundwater, Oecologia 100, 21-28.
O'Malley, C. and Sheldon, F. (1990) Chowilla floodplain biological study.
Nature Conservation Society of South Australia, Adelaide
Roberts, J. and Ludwig, J.A. (1990) Riparian habitats on the Chowilla
floodplain of the River Murray, South Australia, Wetlands (Australia)
9, 13-19
Roberts, J. (2001) Large plants. In Young, W.J.(ed), Rivers as Ecological
Systems - the Murray-Darling Basin, pp. 187-221. Murray-Darling Basin
Commission, Canberra.
Roberts, J. and Marston, F. (2000) Water regime of wetland and floodplain
plants in the Murray-Darling Basin. A sourcebook of ecological knowledge.,
CSIRO Land and Water, Canberra.
Stone, C. and Bacon, P.E. (1994) Relationships among moisture stress,
insect herbivory, foliar cineole content and the growth of river red gum
Eucalyptus camaldulensis. Journal of Applied Ecology 31:
604-612.
Thornburn, P., Walker, G. and Hatton, T. (1992) Are river red gums taking
water from soil, groundwater or streams? Catchments of green: a national
conference on vegetation & water management, conference proceedings
- volume B, 63-68, Greening Australia, Canberra.
Thorburn, P.J. and Walker, G.R. (1994) Variations in stream water uptake
by Eucalyptus camaldulensis with differing access to stream water.
Oecologia 100:293-301.
|

